Hyderabad’s vaccine capacity to soar to 14 billion doses
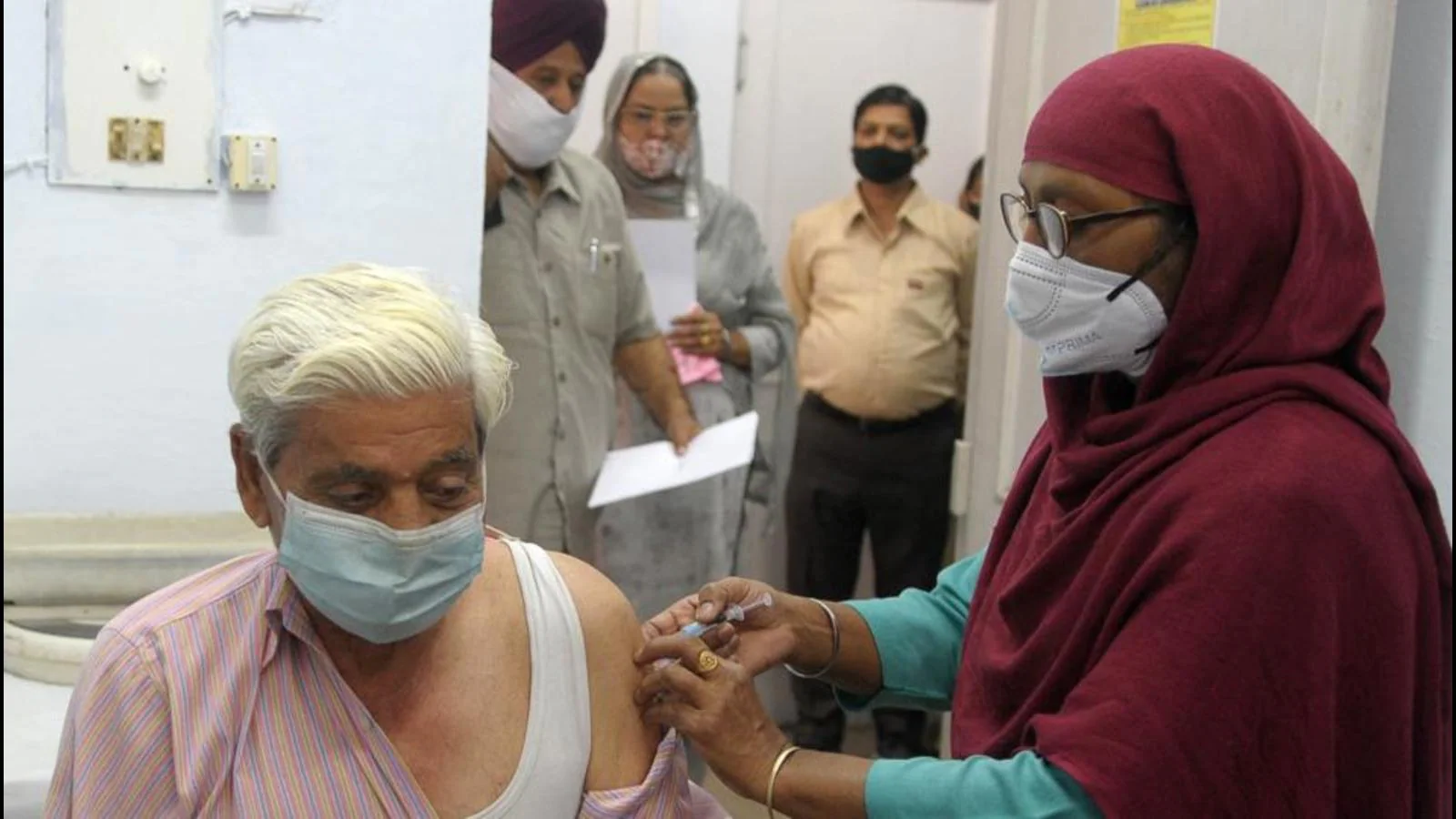
Vaccine makers will invest more than 1,800 crore hospitals in their facilities in Genome Valley.This will strengthen the position of Hyderabad further as ‘World Vaccine Capital’ Hyderabad currently contributes one third of the production of global vaccine with a capacity of around 9 billion doses per year. Investment from this biological E will increase capacity with 5 billion doses to increase cumulative capacity to around 14 billion doses each year.
This expansion is mainly targeted to increase the making of vaccines together with generic and R&D injection.This expansion is expected to create more than 2,500 new jobs.The announcement was made after the Minister of Industry K.T. Rama Rao’s meeting with the leadership of the Biological Implementing Director E Mahima Datla.The company says this investment will be focused on making Janssen Covid vaccines, MR vaccine, PCVA vaccine, typhoid vaccine, covid vaccine, tetanus toxide ampoules, IPV vaccines and pertussis vaccines, fire and biological formulations.
All of these activities will be located in Genome Valley, which is the first organized cluster in India R&D biological science and clean manufacturing activities, with world -class infrastructure facilities in the form of industrial/knowledge parks, special economic zones (KEK), multiple dry and wet laboratories that are rented and incubation facilities.
Genome Valley is home to more than 200 companies with scientific workers consisting of around 15,000 professionals including the presence of global tents such as Novartis, Glaxosmithkline, Ferring Pharma, Chemo, Dupont, Ashland, United States Pharmacopeia, Lonza among many others.Biological E has received funds from the US International Development Finance Corporation (DFC) up to $ 50 million to expand the company’s capacity to produce COVID-19 vaccines.
It has also developed one of the original vaccines for Covid -19 in this country – Corbevax. This vaccine also receives a nod from the controller of the Indian General Drug (DCGI) to authorize the use of emergency for the age group 5-12 years.


















Average Rating